CORMEDIX INC. REPORTS FOURTH QUARTER AND FULL-YEAR 2018 FINANCIAL RESULTS, ANNOUNCES REVERSE STOCK SPLIT AND PROVIDES BUSINESS UPDATE
Conference Call Scheduled for Today at 4:30 p.m. Eastern Time
Berkeley Heights, NJ – Mar. 14, 2019 – CorMedix Inc. (NYSE American: CRMD), a biopharmaceutical company focused on developing and commercializing therapeutic products for the prevention and treatment of infectious and inflammatory disease, today announced financial results for the fourth quarter and full-year ended December 31, 2018 and provided an update on recent business events.
The Company also announced today that its Board of Directors has approved a one-for-five reverse stock split of its common stock that is scheduled to become effective before trading opens on March 26, 2019, after which time the CorMedix common stock will trade on a splitadjusted basis under a new CUSIP number 21900C308. As previously disclosed, at the CorMedix Annual Meeting of Stockholders held on June 26, 2018, the Company’s stockholders approved a proposal authorizing the Company’s Board of Directors to effect a reverse stock split at a ratio of between one-for-five and one-for-ten.
Recent Corporate and Clinical Highlights:
• Announced statistically significant topline results of the interim analysis and full data set of its Phase 3 LOCK-IT-100 Study in Neutrolin. A total of 41 catheter related blood stream infections (CRBSI) was determined by the Clinical Adjudication Committee (CAC) in the full data set, compared with 28 CRBSI cases in the interim analysis. The full data set showed a 71% reduction in the risk of occurrence of CRBSIs compared with the active control of heparin, which is well in excess of the study’s assumed treatment effect size of a 55% reduction and statistically significant with a p-value of 0.0006.
• Announced that the FDA has agreed that the Company can request consideration of Neutrolin for approval under the LPAD (Limited Population Pathway for Antibacterial and Antifungal Drugs) pathway. Per FDA guidance, the formal request for consideration of the LPAD pathway is made as part of the NDA.
• Strengthened the Board of Directors with the appointment of Alan W. Dunton, M.D. as a Director.
• Closed a senior secured convertible debt financing with gross proceeds of $7.5 million with its largest shareholder. The loan has a three-year maturity, carries an interest rate of 10% per annum, and is convertible into the Company’s common stock at $1.50 per share. The loan is callable by the Company beginning in June 2020.
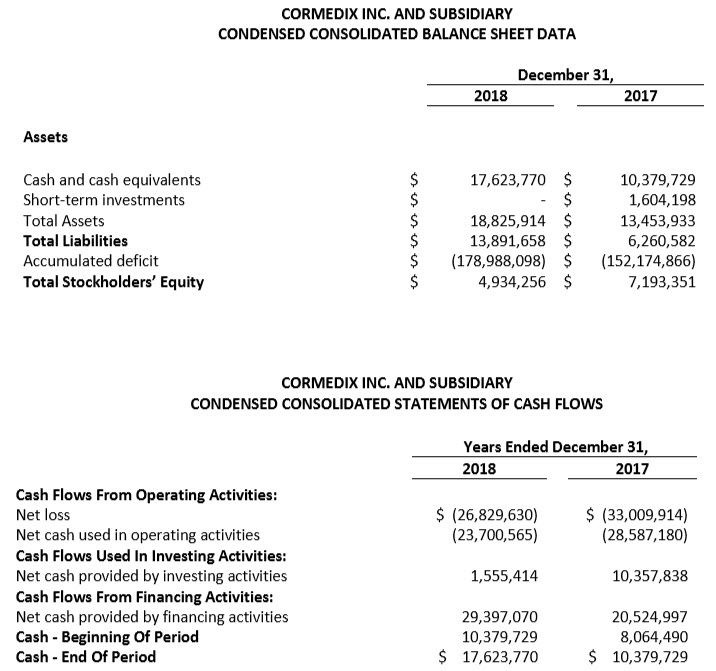
• Cash at December 31, 2018 amounted to $17.6 million.
“We were excited to begin the year with the favorable news that the interim analysis of our LOCKIT-100 study had hit statistical significance of p=0.0034 with its primary endpoint,” said Khoso Baluch, CEO of CorMedix. “We were delighted that the analysis of the full data set supported the interim analysis data, yielding 41 CRBSI cases and a p-value of 0.0006. In our view, these data strongly support our case for Neutrolin’s efficacy as a catheter lock solution in hemodialysis patients. Because the unblinding of the full data set had occurred just prior to our meeting with the FDA, we agreed to provide a detailed analysis of the data to the FDA as soon as possible.”
Mr. Baluch continued, “We were also pleased that the FDA agreed the Company could request consideration of Neutrolin for approval under the LPAD pathway. We believe this strengthens our position that the results of the LOCK-IT-100 study are sufficiently robust to demonstrate safety and effectiveness, and support filing of an NDA for Neutrolin.”
Mr. Baluch concluded, “We successfully progressed the Neutrolin development program during 2018. Now that our cash position has been strengthened and we anticipate that existing cash can fund us into the second quarter of 2020, we believe we are well positioned to make significant progress during 2019. We believe also that because we desire to attract more institutional interest into our stock and need to have available unissued shares to reserve for past transactions and provide for future opportunities, it is a good time to move ahead with the reverse stock split approved by the stockholders last year.”
Reverse Stock Split
The reverse stock split will uniformly affect all issued and outstanding shares of the Company’s common stock. The reverse stock split will not alter any stockholder’s percentage ownership interest in CorMedix, except to the extent that the reverse stock split results in fractional shares. No fractional shares will be issued in connection with the reverse stock split. Stockholders who would otherwise be entitled to receive a fractional share will instead receive one whole share of the Company’s common stock. The par value of the Company’s common stock will remain unchanged at $0.001 per share after the reverse stock split.
The number of shares of common stock available for issuance under the Company’s equity incentive plans and issuable upon the exercise of stock options, warrants and preferred stock outstanding immediately prior to the reverse split will be proportionately affected by the reverse stock split. The exercise prices of the Company’s outstanding options and warrants, and the conversion price of its outstanding preferred stock will be adjusted in accordance with their respective terms.
As a result of the reverse stock split, the number of shares of common stock issued and outstanding will be reduced from approximately 119.0 million to approximately 23.8 million shares. There will be no change to the number of authorized shares.
VStock Transfer, LLC will act as the exchange agent and transfer agent for the reverse stock split. Computershare will provide instructions to stockholders with physical certificates regarding the optional process for exchanging their pre-split stock certificates for post-split stock certificates.
Fourth Quarter 2018 Financial Highlights
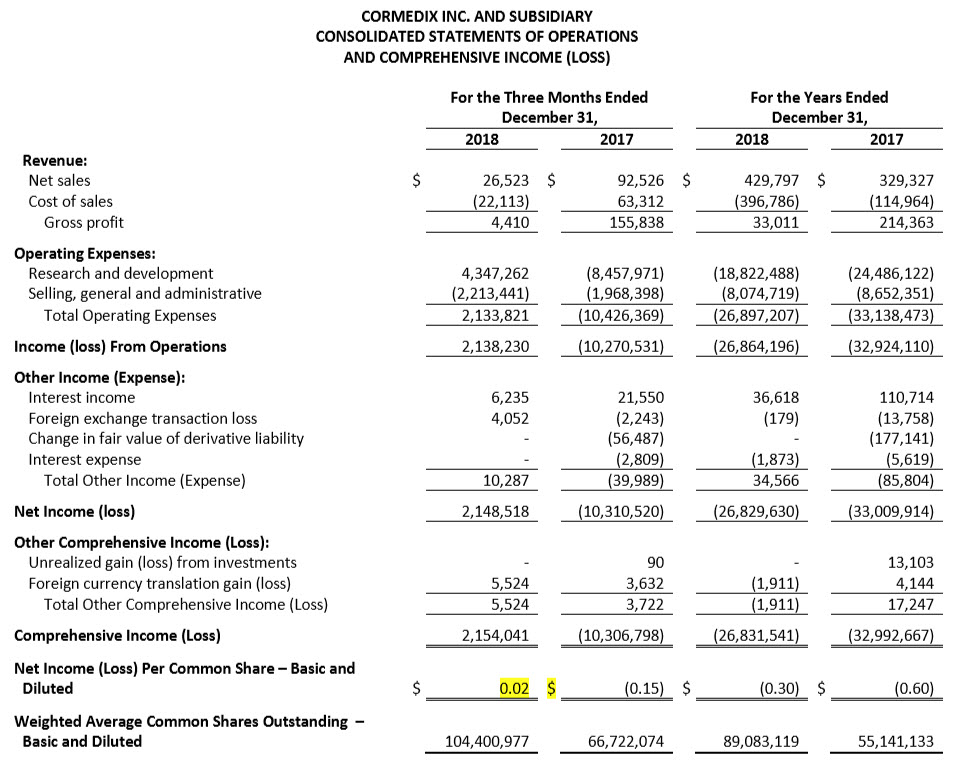
For the fourth quarter 2018, CorMedix recorded a net income of $2.1 million, or $0.0x per share, compared with a net loss of $10.3 million, or $0.15 per share in the fourth quarter 2017, an increase of $12.4 million. The significant improvement in the fourth quarter 2018 was primarily driven by the reversal of clinical trial expenses as a result of the confidential settlement agreement with our CRO signed in November 2018.
Operating expenses in the fourth quarter 2018 were $2.1 million, compared to $10.4 million in the fourth quarter of 2017, a decrease of approximately 120%. This decrease was due primarily to a $12.8 million, or 151% decrease, in R&D expense, while SG&A expenses remained unchanged.
Full-Year 2018 Financial Highlights
For the full-year 2018, CorMedix recorded a net loss of $26.9 million, or $0.30 per share, compared with a net loss of $32.9 million, or $0.60 per share for the full-year 2017. The decrease in the loss in 2018 was primarily driven by decreased costs related to the LOCK-IT-100 clinical study.
Operating expenses for the full-year 2018 were $26.9 million, compared to $33.1 million for the full-year 2017, a decrease of approximately 19%. This decrease was due primarily to a $5.7 million, or 23% decrease, in R&D expense, while SG&A expenses remained unchanged.
Conference Call Information:
The management team of CorMedix will host a conference call and webcast today, March 14, 2019, at 4:30 PM Eastern Time, to discuss recent corporate developments and financial results. Call details and dial-in information is as follows:
Domestic: 877-407-9124 International: 201-689-8584 Passcode: 13688391 Webcast: https://www.investornetwork.com/event/presentation/45037
Replay will be available through March 28, 2019: Domestic: 877-481-4010 International: 919-882-2331 Conference ID: 45037
About CorMedix
CorMedix Inc. is a biopharmaceutical company focused on developing and commercializing therapeutic products for the prevention and treatment of infectious and inflammatory diseases. The Company is focused on developing its lead product Neutrolin®, a novel, non-antibiotic antimicrobial solution designed to prevent costly and dangerous bloodstream infections associated with the use of central venous catheters, currently in Phase 3 development for patients undergoing chronic hemodialysis. Such infections have significant treatment costs and lead to increased morbidity and mortality. Neutrolin has FDA Fast Track status and is designated as a Qualified Infectious Disease Product, which provide the potential for priority review of a marketing application by FDA and allow for a total of ten years of market exclusivity in the event of U.S. approval. Neutrolin is already marketed as a CE Marked product in Europe and other territories. In parallel, CorMedix is leveraging its taurolidine technology to develop a pipeline of antimicrobial medical devices, with active programs in surgical sutures and meshes, and topical hydrogels. The company is also working with top-tier researchers to develop taurolidine-based therapies for rare pediatric cancers. For more information, visit: www.cormedix.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks and uncertainties. All statements, other than statements of historical facts, regarding management’s expectations, beliefs, goals, plans or CorMedix’s prospects, future financial position, financing plans, future revenues and projected costs should be considered forward-looking. Readers are cautioned that actual results may differ materially from projections or estimates due to a variety of important factors, including: risks relating to the effectiveness of the reverse stock split; risks relating to the effect of the reverse stock split on the Company’s stock price and its overall market capitalization; the results of our discussions with the FDA regarding the Neutrolin development path; the resources needed to complete the information required to submit a new drug application for Neutrolin to the FDA; the risks and uncertainties associated with CorMedix’s ability to manage its limited cash resources and the impact on current, planned or future research, including the continued development of Neutrolin and research for additional uses for taurolidine; obtaining additional financing to support CorMedix’s research and development and clinical activities and operations; preclinical results are not indicative of success in clinical trials and might not be replicated in any subsequent studies or trials; and the ability to retain and hire necessary personnel to staff our operations appropriately. These and other risks are described in greater detail in CorMedix’s filings with the SEC, copies of which are available free of charge at the SEC’s website at www.sec.gov or upon request from CorMedix. CorMedix may not actually achieve the goals or plans described in its forward-looking statements, and investors should not place undue reliance on these statements. CorMedix assumes no obligation and does not intend to update these forward-looking statements, except as required by law.
Investor Contact:
Dan Ferry
Managing Director
LifeSci Advisors
617-535-7746